CHAPTER TWO: HISTORICAL DEVELOPMENT OF ATOMIC THEORY CHAPTER TWO: HISTORICAL DEVELOPMENT OF ATOMIC THEORY
Here is a brief and clear Chapter Two on the Historical Development of Atomic Theory:
The idea of the atom has evolved over many centuries as scientists made new discoveries that improved our understanding of matter.
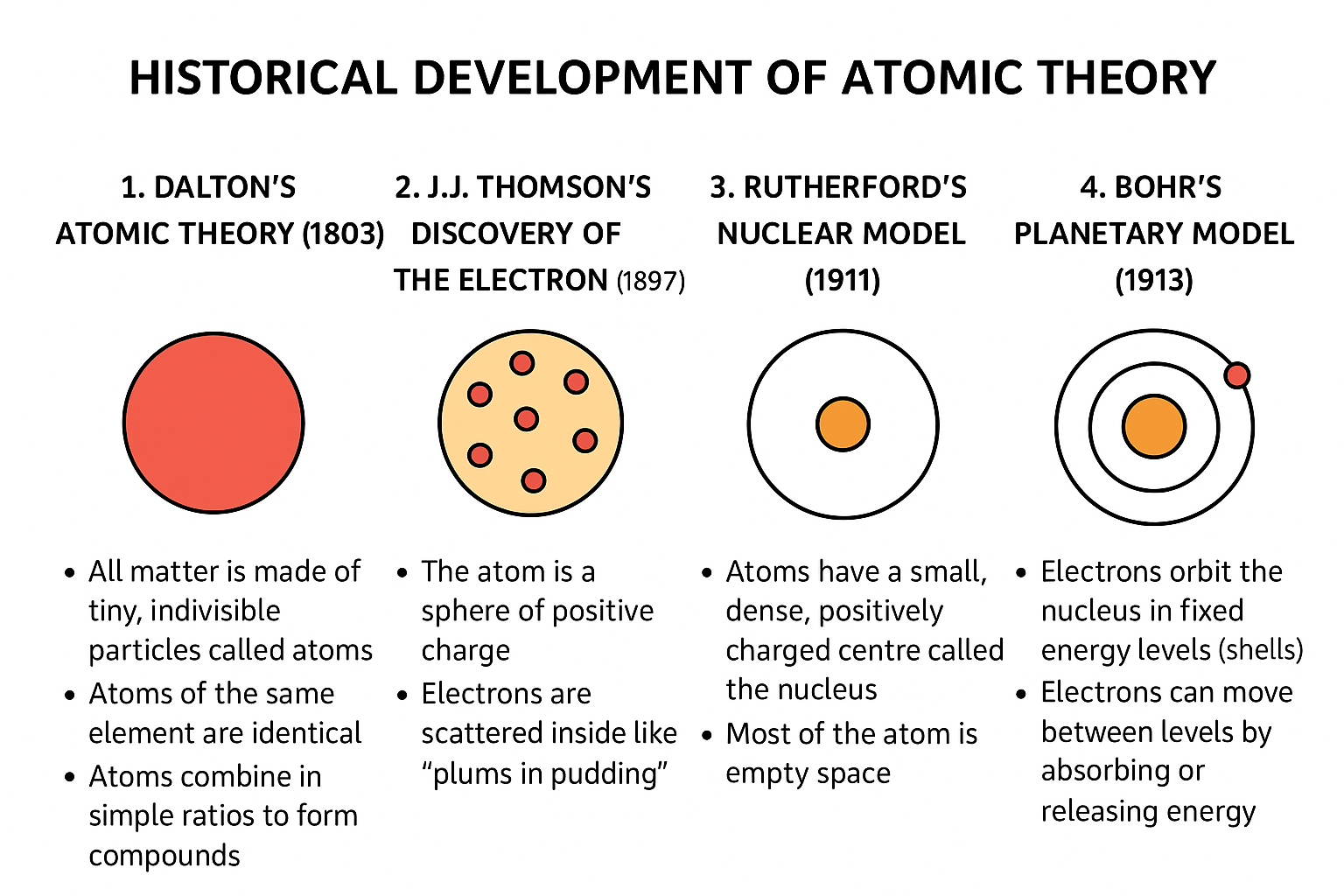
1. Dalton’s Atomic Theory (1803)
John Dalton proposed the first scientific atomic theory. He stated that:
-
All matter is made of tiny, indivisible particles called atoms.
-
Atoms of the same element are identical.
-
Atoms combine in simple ratios to form compounds.
Dalton’s work marked the beginning of modern atomic science.
2. J.J. Thomson’s Discovery of the Electron (1897)
Thomson used cathode ray experiments and discovered the electron, a negatively charged particle.
He proposed the Plum Pudding Model, suggesting that:
-
The atom is a sphere of positive charge
-
Electrons are scattered inside like "plums in pudding"
3. Rutherford’s Nuclear Model (1911)
Ernest Rutherford performed the gold foil experiment. He found that:
-
Atoms have a small, dense, positively charged centre called the nucleus
-
Most of the atom is empty space
This overturned Thomson’s model.
4. Bohr’s Planetary Model (1913)
Niels Bohr improved Rutherford’s model by proposing that:
-
Electrons orbit the nucleus in fixed energy levels (shells)
-
Electrons can move between levels by absorbing or releasing energy
5. The Modern Quantum Mechanical Model
Later scientists (Schrödinger, Heisenberg) discovered that:
-
Electrons do not follow fixed orbits
-
Instead, they exist in regions called orbitals
-
The behaviour of electrons is explained by quantum mechanics
This is the model used today